
SL Paper 2
The rate of the acid-catalysed iodination of propanone can be followed by measuring how the concentration of iodine changes with time.
I2(aq) + CH3COCH3(aq) → CH3COCH2I(aq) + H+(aq) + I−(aq)
Suggest how the change of iodine concentration could be followed.
A student produced these results with [H+] = 0.15 moldm−3. Propanone and acid were in excess and iodine was the limiting reagent.
Determine the relative rate of reaction when [H+] = 0.15 moldm−3.
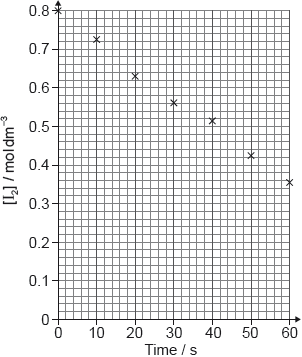
The student then carried out the experiment at other acid concentrations with all other conditions remaining unchanged.
State and explain the relationship between the rate of reaction and the concentration of acid.
Markscheme
use a colorimeter/monitor the change in colour
OR
take samples AND quench AND titrate «with thiosulfate»
Accept change in pH.
Accept change in conductivity.
Accept other suitable methods.
Method must imply “change”.
[1 mark]
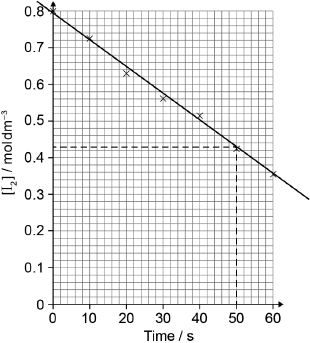
best fit line
relative rate of reaction = « =» 0.0074/7.4 x 10−3
Best fit line required for M1.
M2 is independent of M1.
Accept range from 0.0070 to 0.0080.
[2 marks]
Relationship:
rate of reaction is «directly» proportional to [H+]
OR
rate of reaction [H+]
Explanation:
more frequent collisions/more collisions per unit of time «at greater concentration»
Accept "doubling the concentration doubles the rate".
Do not accept “rate increases as concentration increases”.
Do not accept collisions more likely.
[2 marks]
Examiners report
Sodium thiosulfate solution reacts with dilute hydrochloric acid to form a precipitate of sulfur at room temperature.
Na2S2O3 (aq) + 2HCl (aq) → S (s) + SO2 (g) + 2NaCl (aq) + X
Identify the formula and state symbol of X.
Suggest why the experiment should be carried out in a fume hood or in a well-ventilated laboratory.
The precipitate of sulfur makes the mixture cloudy, so a mark underneath the reaction mixture becomes invisible with time.
10.0 cm3 of 2.00 mol dm-3 hydrochloric acid was added to a 50.0 cm3 solution of sodium thiosulfate at temperature, T1. Students measured the time taken for the mark to be no longer visible to the naked eye. The experiment was repeated at different concentrations of sodium thiosulfate.
Show that the hydrochloric acid added to the flask in experiment 1 is in excess.
Draw the best fit line of against concentration of sodium thiosulfate on the axes provided.
A student decided to carry out another experiment using 0.075 mol dm-3 solution of sodium thiosulfate under the same conditions. Determine the time taken for the mark to be no longer visible.
An additional experiment was carried out at a higher temperature, T2.
(i) On the same axes, sketch Maxwell–Boltzmann energy distribution curves at the two temperatures T1 and T2, where T2 > T1.
(ii) Explain why a higher temperature causes the rate of reaction to increase.
Suggest one reason why the values of rates of reactions obtained at higher temperatures may be less accurate.
Markscheme
H2O AND (l)
Do not accept H2O (aq).
SO2 (g) is an irritant/causes breathing problems
OR
SO2 (g) is poisonous/toxic
Accept SO2 (g) is acidic, but do not accept “causes acid rain”.
Accept SO2 (g) is harmful.
Accept SO2 (g) has a foul/pungent smell.
n(HCl) = «dm3 × 2.00 mol dm-3 =» 0.0200 / 2.00 × 10-2«mol»
AND
n(Na2S2O3) = «dm3 × 0.150 mol × dm-3 =» 0.00750 / 7.50 × 10-3 «mol»
0.0200 «mol» > 0.0150 «mol»
OR
2.00 × 10-2«mol» > 2 × 7.50 × 10-3 «mol»
OR
× 2.00 × 10-2 «mol» > 7.50 × 10-3 «mol»
Accept answers based on volume of solutions required for complete reaction.
Award [2] for second marking point.
Do not award M2 unless factor of 2 (or half) is used.
five points plotted correctly
best fit line drawn with ruler, going through the origin
22.5 × 10-3 «s-1»
«Time = =» 44.4 «s»
Award [2] for correct final answer.
Accept value based on candidate’s graph.
Award M2 as ECF from M1.
Award [1 max] for methods involving taking mean of appropriate pairs of values.
Award [0] for taking mean of pairs of time values.
Award [2] for answers between 42.4 and 46.4 «s».
(i)
correctly labelled axes
peak of T2 curve lower AND to the right of T1 curve
Accept “probability «density» / number of particles / N / fraction” on y-axis.
Accept “kinetic E/KE/EK” but not just “Energy/E” on x-axis.
(ii)
greater proportion of molecules have E ≥ Ea or E > Ea
OR
greater area under curve to the right of the Ea
greater frequency of collisions «between molecules»
OR
more collisions per unit time/second
Accept more molecules have energy greater than Ea.
Do not accept just “particles have greater kinetic energy”.
Accept “rate/chance/probability/likelihood/” instead of “frequency”.
Accept suitably shaded/annotated diagram.
Do not accept just “more collisions”.
shorter reaction time so larger «%» error in timing/seeing when mark disappears
Accept cooling of reaction mixture during course of reaction.
Examiners report
Hydrogen peroxide can react with methane and oxygen to form methanol. This reaction can occur below 50°C if a gold nanoparticle catalyst is used.
Methanol is usually manufactured from methane in a two-stage process.
CH4 (g) + H2O (g) CO (g) + 3H2 (g)
CO (g) + 2H2 (g) CH3OH (l)
Consider the first stage of the reaction.
CH4 (g) + H2O (g) CO (g) + 3H2 (g)
The diagram shows the Maxwell-Boltzmann curve for the uncatalyzed reaction.
Draw a distribution curve at a lower temperature (T2) and show on the diagram how the addition of a catalyst enables the reaction to take place more rapidly than at T1.
The hydrogen peroxide could cause further oxidation of the methanol. Suggest a possible oxidation product.
Determine the overall equation for the production of methanol.
8.00 g of methane is completely converted to methanol. Calculate, to three significant figures, the final volume of hydrogen at STP, in dm3. Use sections 2 and 6 of the data booklet.
Determine the enthalpy change, ΔH, in kJ. Use section 11 of the data booklet.
Bond enthalpy of CO = 1077 kJ mol−1.
State the expression for Kc for this stage of the reaction.
State and explain the effect of increasing temperature on the value of Kc.
Markscheme
curve higher AND to left of T1 ✔
new/catalysed Ea marked AND to the left of Ea of curve T1 ✔
Do not penalize curve missing a label, not passing exactly through the origin, or crossing x-axis after Ea.
Do not award M1 if curve drawn shows significantly more/less molecules/greater/smaller area under curve than curve 1.
Accept Ea drawn to T1 instead of curve drawn as long as to left of marked Ea.
methanoic acid/HCOOH/CHOOH
OR
methanal/HCHO ✔
Accept “carbon dioxide/CO2”.
CH4(g) + H2O(g) CH3OH(l) + H2(g) ✔
Accept arrow instead of equilibrium sign.
amount of methane = « = » 0.498 «mol» ✔
amount of hydrogen = amount of methane / 0.498 «mol» ✔
volume of hydrogen = «0.498 mol × 22.7 dm3 mol−1 = » 11.3 «dm3» ✔
Award [3] for final correct answer.
Award [2 max] for 11.4 «dm3 due to rounding of mass to 16/moles to 0.5. »
Σbonds broken = 4 × 414 «kJ» + 2 × 463 «kJ» / 2582 «kJ» ✔
Σbonds formed = 1077 «kJ» + 3 × 436 «kJ» / 2385 «kJ» ✔
ΔH «= Σbonds broken − Σbonds formed =( 2582 kJ − 2385 kJ)» = «+»197«kJ» ✔
Award [3] for final correct answer.
Award [2 Max] for final answer of −197 «kJ»
✔
Kc increases AND «forward» reaction endothermic ✔
Examiners report
The thermal decomposition of dinitrogen monoxide occurs according to the equation:
2N2O (g) → 2N2 (g) + O2 (g)
The reaction can be followed by measuring the change in total pressure, at constant temperature, with time.
The x-axis and y-axis are shown with arbitrary units.
Explain why, as the reaction proceeds, the pressure increases by the amount shown.
Outline, in terms of collision theory, how a decrease in pressure would affect the rate of reaction.
The experiment is repeated using the same amount of dinitrogen monoxide in the same apparatus, but at a lower temperature.
Sketch, on the axes in question 2, the graph that you would expect.
The experiment gave an error in the rate because the pressure gauge was inaccurate. Outline whether repeating the experiment, using the same apparatus, and averaging the results would reduce the error.
The graph below shows the Maxwell–Boltzmann distribution of molecular energies at a particular temperature.
The rate at which dinitrogen monoxide decomposes is significantly increased by a metal oxide catalyst.
Annotate and use the graph to outline why a catalyst has this effect.
Markscheme
increase in the amount/number of moles/molecules «of gas» [✔]
from 2 to 3/by 50 % [✔]
«rate of reaction decreases»
concentration/number of molecules in a given volume decreases
OR
more space between molecules [✔]
collision rate/frequency decreases
OR
fewer collisions per second/unit time [✔]
Note: Do not accept just “larger space/volume” for M1.
smaller initial gradient [✔]
initial pressure is lower AND final pressure of gas lower «by similar factor» [✔]
no AND it is a systematic error/not a random error
OR
no AND «a similar magnitude» error would occur every time [✔]
catalysed and uncatalysed Ea marked on graph AND with the catalysed being at lower energy [✔]
«for catalysed reaction» greater proportion of/more molecules have E ≥ Ea / E > Ea
OR
«for catalysed reaction» greater area under curve to the right of the Ea [✔]
Note: Accept “more molecules have the activation energy”.
Examiners report
About a quarter of the candidates gave the full answer. Some only gained the first marking point (M1) by recognizing the increase in the number of moles of gas. Some candidates wrote vague answers that did not receive credit such as “pressure increases as more gaseous products form” without explicitly recognizing that the reactants have fewer moles of gas than the products. Some candidates mistook it for a system at equilibrium when the pressure stops changing (although a straight arrow is shown in the equation). A teacher commented that the wording of the question was rather vague “not clear if question is asking about stoichiometry (i.e. how 200 & 300 connect to coefficients) or rates (i.e. explain graph shape)”. We did not see a discussion of the slope of the graph with time and most candidates understood the question as it was intended.
More than half of the candidates obtained the mark allocated for “less frequent collisions” at lower pressure, but only strong candidates explained that this was due to the lower concentration or increased spacing between molecules. Some candidates talked about a decrease in kinetic energy and they did not show a good understanding of collision theory. Some candidates lost M1 for stating “fewer collisions” without reference to time or probability.
This was a challenging question. Candidates usually obtained only one of the two marks allocated for the answer. Most of them scored the mark for a lower initial slope at low temperature, while others scored a mark for sketching their curve below the original curve as all pressures (initial and final) will be lower at the lower temperature. A teacher commented that the wording was unclear “sketch on the axes in question 2”, and it would have been better to label the graph instead.
This question was well answered by nearly 70 % of the candidates reflecting a good understanding of the impact of systematic errors. Some students did not gain the mark because of an incomplete answer. The question raised much debate among teachers. They worried if the error was clearly a systematic one. However, a high proportion of candidates had very clear and definite answers. In Spanish and French, the wording was a bit ambiguous which caused the markscheme in these languages to be more opened.
This question discriminated very well between high-scoring and low-scoring candidates. About half of the candidates annotated the Maxwell-Boltzmann distribution to show the effect of the catalyst. Some left it blank and some sketched a new distribution that would be obtained at a higher temperature instead. The majority of candidates knew that the catalyst provided an alternative route with lower Ea but only stronger candidates related it to the annotation of the graph and used the accurate language needed to score M2. A common mistake was stating that molecules have higher kinetic energy when a catalyst is added.
Magnesium reacts with sulfuric acid:
Mg(s) + H2SO4(aq) → MgSO4(aq) + H2(g)
The graph shows the results of an experiment using excess magnesium ribbon and dilute sulfuric acid.
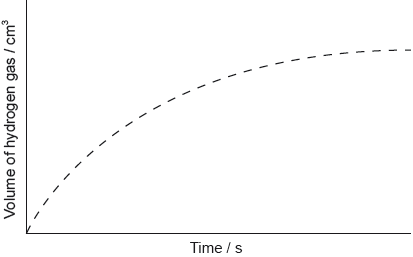
Outline why the rate of the reaction decreases with time.
Sketch, on the same graph, the expected results if the experiment were repeated using powdered magnesium, keeping its mass and all other variables unchanged.
Nitrogen dioxide and carbon monoxide react according to the following equation:
NO2(g) + CO(g) NO(g) + CO2(g) ΔH = –226 kJ
Calculate the activation energy for the reverse reaction.
State the equation for the reaction of NO2 in the atmosphere to produce acid deposition.
Markscheme
concentration of acid decreases
OR
surface area of magnesium decreases
Accept “less frequency/chance/rate/probability/likelihood of collisions”.
Do not accept just “less acid” or “less magnesium”.
Do not accept “concentrations of reagents decrease”.
[1 mark]
curve starting from origin with steeper gradient AND reaching same maximum volume
[1 mark]
«Ea(rev) = 226 + 132 =» 358 «kJ»
Do not accept –358.
[1 mark]
2NO2(g) + H2O(l) → HNO3(aq) + HNO2(aq)
OR
2NO2(g) + 2H2O(l) + O2(g) → 4HNO3(aq)
Accept ionised forms of the acids.
[1 mark]
Examiners report
Graphing is an important tool in the study of rates of chemical reactions.
Excess hydrochloric acid is added to lumps of calcium carbonate. The graph shows the volume of carbon dioxide gas produced over time.
Sketch a Maxwell–Boltzmann distribution curve for a chemical reaction showing the activation energies with and without a catalyst.
Sketch a curve on the graph to show the volume of gas produced over time if the same mass of crushed calcium carbonate is used instead of lumps. All other conditions remain constant.
State and explain the effect on the rate of reaction if ethanoic acid of the same concentration is used in place of hydrochloric acid.
Outline why pH is more widely used than [H+] for measuring relative acidity.
Outline why H3PO4/HPO42− is not a conjugate acid-base pair.
Markscheme
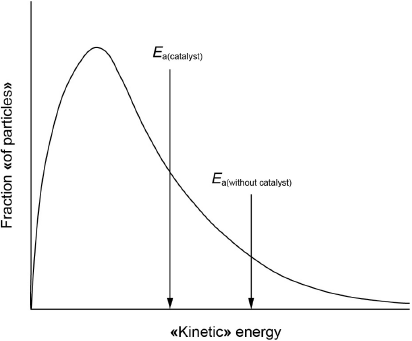
both axes correctly labelled
correct shape of curve starting at origin
Ea(catalyst) < Ea(without catalyst) on x-axis
M1:
Accept “speed” for x-axis label.
Accept “number of particles”, “N”, “frequency” or “probability «density»” for y-axis label.
Do not accept “potential energy” for x-axis label.
M2:
Do not accept a curve that touches the x-axis at high energy.
Do not award M2 if two curves are drawn.
M3:
Ignore any shading under the curve.
[3 marks]
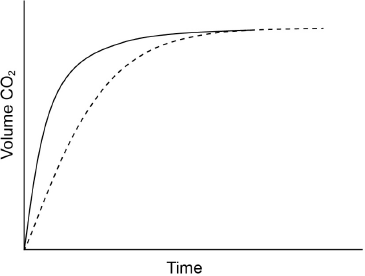
curve starting from origin with steeper gradient AND reaching same maximum volume
[1 mark]
rate decreases
OR
slower reaction
«ethanoic acid» partially dissociated/ionized «in solution/water»
OR
lower [H+]
Accept “weak acid” or “higher pH”.
[2 marks]
«pH» converts «wide range of [H+]» into simple «log» scale/numbers
OR
«pH» avoids need for exponential/scientific notation
OR
«pH» converts small numbers into values «typically» between 0/1 and 14
OR
«pH» allows easy comparison of values of [H+]
Accept “uses values between 0/1 and 14”.
Do not accept “easier to use”.
Do not accept “easier for calculations”.
[1 mark]
«species» do not differ by a «single» proton/H+
OR
conjugate base of H3PO4 is H2PO4– «not HPO42–»
OR
conjugate acid of HPO42– is H2PO4– «not H3PO4»
Do not accept “hydrogen/H” for “H+/proton”.
[1 mark]
Examiners report
1-chloropentane reacts with aqueous sodium hydroxide.
The reaction was repeated at a lower temperature.
Identify the type of reaction.
Outline the role of the hydroxide ion in this reaction.
Suggest, with a reason, why 1-iodopentane reacts faster than 1-chloropentane under the same conditions. Use section 11 of the data booklet for consistency.
Sketch labelled Maxwell–Boltzmann energy distribution curves at the original temperature (T1) and the new lower temperature (T2).
Explain the effect of lowering the temperature on the rate of the reaction.
Markscheme
«nucleophilic» substitution/SN2 ✔
Do not accept if “electrophilic” or “free radical” substitution is stated.
«acts as a» nucleophile/Lewis base
OR
donates/provides lone pair «of electrons»
OR
attacks the «partially» positive carbon ✔
bond enthalpy C–I lower than C–Cl
OR
C–I bond weaker than C–Cl ✔
«weaker bond» broken more easily/with less energy
OR
lower Ea «for weaker bonds» ✔
Accept the bond enthalpy values for C–I and C–Cl for M1.
peak at T1 to right of AND lower than T2 ✔
lines begin at origin AND T1 must finish above T2 ✔
«rate is» lower AND «average» kinetic energy of molecules is lower
OR
«rate is» lower AND less frequent collisions
OR
«rate is» lower AND fewer collisions per unit time ✔
«rate is» lower AND fewer/smaller fraction of molecules/collisions have the E ≥ Ea ✔
Lower «rate» needs to be mentioned once only.
Do not accept “fewer collisions” without reference to time/frequency/probability for M1.
Examiners report
Calcium carbonate reacts with hydrochloric acid.
CaCO3(s) + 2HCl(aq) → CaCl2(aq) + H2O(l) + CO2(g)
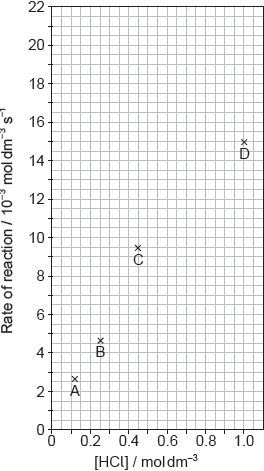
The results of a series of experiments in which the concentration of HCl was varied are shown below.
Outline two ways in which the progress of the reaction can be monitored. No practical details are required.
Suggest why point D is so far out of line assuming human error is not the cause.
Suggest the relationship that points A, B and C show between the concentration of the acid and the rate of reaction.
Markscheme
Any two of:
loss of mass «of reaction mixture/CO2»
«increase in» volume of gas produced
change of conductivity
change of pH
change in temperature
Do not accept “disappearance of calcium carbonate”.
Do not accept “gas bubbles”.
Do not accept “colour change” or “indicator”.
[2 marks]
reaction is fast at high concentration AND may be difficult to measure accurately
OR
so many bubbles of CO2 produced that inhibit contact of HCl(aq) with CaCO3(s)
OR
insufficient change in conductivity/pH at high concentrations
OR
calcium carbonate has been used up/is limiting reagent/there is not enough calcium carbonate «to react with the high concentration of HCl»
OR
HCl is in excess
OR
so many bubbles of CO2 produced that inhibit contact of HCl(aq) with CaCO3(s)
[1 mark]
«directly» proportional
Accept “first order” or “linear”.
Do not accept “rate increases as concentration increases” or “positive correlation”
[1 mark]
Examiners report
A student titrated an ethanoic acid solution, CH3COOH (aq), against 50.0 cm3 of 0.995 mol dm–3 sodium hydroxide, NaOH (aq), to determine its concentration.
The temperature of the reaction mixture was measured after each acid addition and plotted against the volume of acid.
Curves X and Y were obtained when a metal carbonate reacted with the same volume of ethanoic acid under two different conditions.
Using the graph, estimate the initial temperature of the solution.
Determine the maximum temperature reached in the experiment by analysing the graph.
Calculate the concentration of ethanoic acid, CH3COOH, in mol dm–3.
Determine the heat change, q, in kJ, for the neutralization reaction between ethanoic acid and sodium hydroxide.
Assume the specific heat capacities of the solutions and their densities are those of water.
Calculate the enthalpy change, ΔH, in kJ mol–1, for the reaction between ethanoic acid and sodium hydroxide.
Explain the shape of curve X in terms of the collision theory.
Suggest one possible reason for the differences between curves X and Y.
Markscheme
21.4 °C
Accept values in the range of 21.2 to 21.6 °C.
29.0 «°C»
Accept range 28.8 to 29.2 °C.
ALTERNATIVE 1
«volume CH3COOH =» 26.0 «cm3»
«[CH3COOH] = 0.995 mol dm–3 \( \times \frac{{50.0\,{\text{cm3}}}}{{26.0\,{\text{cm3}}}} = \)» 1.91 «mol dm−3»
ALTERNATIVE 2
«n(NaOH) =0.995 mol dm–3 x 0.0500 dm3 =» 0.04975 «mol»
«[CH3COOH] = dm3 =» 1.91 «mol dm–3»
Accept values of volume in range 25.5 to 26.5 cm3.
Award [2] for correct final answer.
«total volume = 50.0 + 26.0 =» 76.0 cm3 AND «temperature change 29.0 – 21.4 =» 7.6 «°C»
«q = 0.0760 kg x 4.18 kJ kg–1 K–1 x 7.6 K =» 2.4 «kJ»
Award [2] for correct final answer.
«n(NaOH) = 0.995 mol dm–3 x 0.0500 dm3 =» 0.04975 «mol»
OR
«n(CH3COOH) = 1.91 mol dm–3 x 0.0260 dm3 =» 0.04966 «mol»
«ΔH = » –48 / –49 «kJ mol–1»
Award [2] for correct final answer.
Negative sign is required for M2.
«initially steep because» greatest concentration/number of particles at start
OR
«slope decreases because» concentration/number of particles decreases
volume produced per unit of time depends on frequency of collisions
OR
rate depends on frequency of collisions
mass/amount/concentration of metal carbonate more in X
OR
concentration/amount of CH3COOH more in X
Examiners report
When dinitrogen pentoxide, N2O5, is heated the colourless gas undergoes thermal decomposition to produce brown nitrogen dioxide:
N2O5 (g) → 2NO2 (g) + O2 (g)
Data for the decomposition at constant temperature is given.
Suggest how the extent of decomposition could be measured.
Plot the missing point on the graph and draw the best-fit line.
Deduce the relationship between the concentration of N2O5 and the rate of reaction.
Outline why increasing the concentration of N2O5 increases the rate of reaction.
Markscheme
use colorimeter
OR
change in colour
OR
change in volume
OR
change in pressure ✔
Accept suitable instruments, e.g. pressure probe/oxygen sensor.
point correct ✔
straight line passing close to all points AND through origin ✔
Accept free hand drawn line as long as attempt to be linear and meets criteria for M2.
« rate of reaction is directly» proportional to/∝[N2O5]
OR
doubling concentration doubles rate ✔
Do not accept “rate increases as concentration increases”/ positive correlation
Accept linear
greater frequency of collisions «as concentration increases»
OR
more collisions per unit time «as concentration increases» ✔
Accept “rate/chance/probability/likelihood” instead of “frequency”.
Do not accept just “more collisions”.
Examiners report
Nickel catalyses the conversion of propanone to propan-2-ol.
Outline how a catalyst increases the rate of reaction.
Explain why an increase in temperature increases the rate of reaction.
Discuss, referring to intermolecular forces present, the relative volatility of propanone and propan-2-ol.
The diagram shows an unlabelled voltaic cell for the reaction
Label the diagram with the species in the equation.
Suggest a metal that could replace nickel in a new half-cell and reverse the electron flow. Use section 25 of the data booklet.
Describe the bonding in metals.
Nickel alloys are used in aircraft gas turbines. Suggest a physical property altered by the addition of another metal to nickel.
Markscheme
provides an alternative pathway/mechanism AND lower ✔
Accept description of how catalyst lowers (e.g. “reactants adsorb on surface «of catalyst»”, “reactant bonds weaken «when adsorbed»”).
more/greater proportion of molecules with ✔
greater frequency/probability/chance of collisions «between the molecules»
OR
more collision per unit of time/second ✔
hydrogen bonding/bonds «and dipole–dipole and London/dispersion forces are present in» propan-2-ol ✔
dipole–dipole «and London/dispersion are present in» propanone ✔
propan-2-ol less volatile AND hydrogen bonding/bonds stronger «than dipole–dipole »
OR
propan-2-ol less volatile AND «sum of all» intermolecular forces stronger ✔
✔
Accept OR .
electrostatic attraction ✔
between «a lattice of» metal/positive ions/cations AND «a sea of» delocalized electrons ✔
Accept “mobile/free electrons”.
Any of:
malleability/hardness
OR
«tensile» strength/ductility
OR
density
OR
thermal/electrical conductivity
OR
melting point
OR
thermal expansion ✔
Do not accept corrosion/reactivity or any chemical property.
Accept other specific physical properties.
Examiners report
A straight-forward question, however, half of the candidates only mentioned the lower activation energy and did not mention that this is through an alternative mechanism, so did not score the mark.
Half of the candidates gained the mark about the increased frequency of collision. Fewer candidates also clarified that a larger proportion of molecules have the activation energy.
Most candidates had the correct structure in their answers identifying the type of intermolecular forces in each compound and then comparing the strength of the two and reaching a conclusion. Some candidates did not know what was meant by volatile. Some candidates stated London dispersion forces in propanone instead of dipole-dipole.
60% of the candidates obtained the mark. Some candidates labelled the electrodes as ions indicating they do not understand the structure of a voltaic cell.
70% of the candidates answered correctly. The common mistake was to select a more reactive metal instead.
The mean mark on the question was 1.0 out of 2 marks. Mistakes included not mentioning the 'electrostatic attraction' and talking about 'nuclei attracting the delocalised electrons'. The weakest candidates discussed aspects of ionic and/or covalent bonding.
80% obtained the mark. Many candidates wrote more than one property, which should be discouraged. Incorrect answers included chemical properties such as reactivity.
3.26 g of iron powder are added to 80.0 cm3 of 0.200 mol dm−3 copper(II) sulfate solution. The following reaction occurs:
Fe (s) + CuSO4 (aq) → FeSO4 (aq) + Cu (s)
Determine the limiting reactant showing your working.
The mass of copper obtained experimentally was 0.872 g. Calculate the percentage yield of copper.
The reaction was carried out in a calorimeter. The maximum temperature rise of the solution was 7.5 °C.
Calculate the enthalpy change, ΔH, of the reaction, in kJ, assuming that all the heat released was absorbed by the solution. Use sections 1 and 2 of the data booklet.
State another assumption you made in (b)(i).
The only significant uncertainty is in the temperature measurement.
Determine the absolute uncertainty in the calculated value of ΔH if the uncertainty in the temperature rise was ±0.2 °C.
Sketch a graph of the concentration of iron(II) sulfate, FeSO4, against time as the reaction proceeds.
Outline how the initial rate of reaction can be determined from the graph in part (c)(i).
Explain, using the collision theory, why replacing the iron powder with a piece of iron of the same mass slows down the rate of the reaction.
Markscheme
nCuSO4 «= 0.0800 dm3 × 0.200 mol dm–3» = 0.0160 mol AND
nFe «» = 0.0584 mol ✔
CuSO4 is the limiting reactant ✔
Do not award M2 if mole calculation is not shown.
ALTERNATIVE 1:
«0.0160 mol × 63.55 g mol–1 =» 1.02 «g» ✔
«» 85.5 «%» ✔
ALTERNATIVE 2:
«» 0.0137 «mol» ✔
«» 85.6 «%» ✔
Accept answers in the range 85–86 %.
Award [2] for correct final answer.
ALTERNATIVE 1:
q = «80.0 g × 4.18 J g–1 K–1 × 7.5 K =» 2.5 × 103 «J»/2.5 «kJ» ✔
«per mol of CuSO4 = kJ mol–1»
«for the reaction» ΔH = –1.6 × 102 «kJ» ✔
ALTERNATIVE 2:
q = «80.0 g × 4.18 J g–1 K–1 × 7.5 K =» 2.5 × 103 «J»/2.5 «kJ» ✔
«nCu = = 0.0137 mol»
«per mol of CuSO4 = kJ mol–1»
«for the reaction» ΔH = –1.8 × 102 «kJ» ✔
Award [2] for correct final answer.
density «of solution» is 1.00 g cm−3
OR
specific heat capacity «of solution» is 4.18 J g−1 K−1/that of «pure» water
OR
reaction goes to completion
OR
iron/CuSO4 does not react with other substances ✔
The mark for “reaction goes to completion” can only be awarded if 0.0160 mol was used in part (b)(i).
Do not accept “heat loss”.
ALTERNATIVE 1:
«» 3 %/0.03 ✔
«0.03 × 160 kJ» = «±» 5 «kJ» ✔
ALTERNATIVE 2:
«» 3 %/0.03 ✔
«0.03 × 180 kJ» = «±» 5 «kJ» ✔
Accept values in the range 4.1–5.5 «kJ».
Award [2] for correct final answer.
initial concentration is zero AND concentration increases with time ✔
decreasing gradient as reaction proceeds ✔
«draw a» tangent to the curve at time = 0 ✔
«rate equals» gradient/slope «of the tangent» ✔
Accept suitable diagram.
piece has smaller surface area ✔
lower frequency of collisions
OR
fewer collisions per second/unit time ✔
Accept “chance/probability” instead of “frequency”.
Do not accept just “fewer collisions”.
Examiners report
Magnesium is a reactive metal often found in alloys.
Organomagnesium compounds can react with carbonyl compounds. One overall equation is:
Compound B can also be prepared by reacting an alkene with water.
Iodomethane is used to prepare CH3Mg. It can also be converted into methanol:
CH3 + HO– → CH3OH + –
Magnesium can be produced by the electrolysis of molten magnesium chloride.
Write the half-equation for the formation of magnesium.
Suggest an experiment that shows that magnesium is more reactive than zinc, giving the observation that would confirm this.
State the name of Compound A, applying International Union of Pure and Applied Chemistry (IUPAC) rules.
Identify the strongest force between the molecules of Compound B.
Draw the structural formula of the alkene required.
Deduce the structural formula of the repeating unit of the polymer formed from this alkene.
Deduce what would be observed when Compound B is warmed with acidified aqueous potassium dichromate (VI).
Identify the type of reaction.
Outline the requirements for a collision between reactants to yield products.
The polarity of the carbon–halogen bond, C–X, facilitates attack by HO–.
Outline, giving a reason, how the bond polarity changes going down group 17.
Markscheme
Mg2+ + 2 e- → Mg ✔
Do not penalize missing charge on electron.
Accept equation with equilibrium arrows.
Alternative 1
put Mg in Zn2+(aq) ✔
Zn/«black» layer forms «on surface of Mg» ✔
Award [1 max] for “no reaction when Zn placed in Mg2+(aq)”.
Alternative 2
place both metals in acid ✔
bubbles evolve more rapidly from Mg
OR
Mg dissolves faster ✔
Alternative 3
construct a cell with Mg and Zn electrodes ✔
bulb lights up
OR
shows (+) voltage
OR
size/mass of Mg(s) decreases «over time»
OR
size/mass of Zn increases «over time»
Accept “electrons flow from Mg to Zn”.
Accept Mg is negative electrode/anode
OR
Zn is positive electrode/cathode
Accept other correct methods.
propanone ✔
Accept 2-propanone and propan-2-one.
hydrogen bonds ✔
Do not penalize missing brackets or n.
Do not award mark if continuation bonds are not shown.
no change «in colour/appearance/solution» ✔
«nucleophilic» substitution
OR
SN2 ✔
Accept “hydrolysis”.
Accept SN1
energy/E ≥ activation energy/Ea ✔
correct orientation «of reacting particles»
OR
correct geometry «of reacting particles» ✔
decreases/less polar AND electronegativity «of the halogen» decreases ✔
Accept “decreases” AND a correct comparison of the electronegativity of two halogens.
Accept “decreases” AND “attraction for valence electrons decreases”.
Examiners report
Unfortunately, only 40% of the students could write this quite straightforward half equation.
Many candidates gained some credit by suggesting voltaic cell or a displacement reaction, but most could not gain the second mark and the reason was often a failure to be able to differentiate between "what occurs" and "what is observed".
Even though superfluous numbers (2-propanone, propan-2-one) were overlooked, only about half of the students could correctly name this simple molecule.
Probably just over half the students correctly identified hydrogen bonding, with dipole-dipole being the most common wrong answer, though a significant number identified an intramolecular bond.
Few candidates could correctly eliminate water to deduce the identity of the required reactant.
Correct answers to this were very scarce and even when candidates had an incorrect alkene for the previous part, they were unable to score an ECF mark, by deducing the formula of the polymer it would produce.
Some students deduced that, as it was a tertiary alcohol, there would be no reaction, but almost all were lucky that this was accepted as well as the correct observation - "it would remain orange".
About a quarter of the students identified this as a substitution reaction, though quite a number then lost the mark by incorrectly stating it was either "free radical" or "electrophilic". A very common wrong answer was "displacement" or "single displacement" and this makes one wonder whether this terminology is being taught instead of substitution
Generally well done with the vast majority of students correctly citing "correct orientation" and many only failed to gain the second mark through failing to equate the energy required to the activation energy.
Another question that was not well answered with probably only a quarter of candidates stating that the polarity would decrease because of decreasing electronegativity down the group.
Limestone can be converted into a variety of useful commercial products through the lime cycle. Limestone contains high percentages of calcium carbonate, CaCO3.
The second step of the lime cycle produces calcium hydroxide, Ca(OH)2.
Calcium hydroxide reacts with carbon dioxide to reform calcium carbonate.
Ca(OH)2 (aq) + CO2 (g) → CaCO3 (s) + H2O (l)
Calcium carbonate is heated to produce calcium oxide, CaO.
CaCO3 (s) → CaO (s) + CO2 (g)
Calculate the volume of carbon dioxide produced at STP when 555 g of calcium carbonate decomposes. Use sections 2 and 6 of the data booklet.
Thermodynamic data for the decomposition of calcium carbonate is given.
Calculate the enthalpy change of reaction, ΔH, in kJ, for the decomposition of calcium carbonate.
The potential energy profile for a reaction is shown. Sketch a dotted line labelled “Catalysed” to indicate the effect of a catalyst.
Outline why a catalyst has such an effect.
Write the equation for the reaction of Ca(OH)2 (aq) with hydrochloric acid, HCl (aq).
Determine the volume, in dm3, of 0.015 mol dm−3 calcium hydroxide solution needed to neutralize 35.0 cm3 of 0.025 mol dm−3 HCl (aq).
Saturated calcium hydroxide solution is used to test for carbon dioxide. Calculate the pH of a 2.33 × 10−2 mol dm−3 solution of calcium hydroxide, a strong base.
Determine the mass, in g, of CaCO3 (s) produced by reacting 2.41 dm3 of 2.33 × 10−2 mol dm−3 of Ca(OH)2 (aq) with 0.750 dm3 of CO2 (g) at STP.
2.85 g of CaCO3 was collected in the experiment in e(i). Calculate the percentage yield of CaCO3.
(If you did not obtain an answer to e(i), use 4.00 g, but this is not the correct value.)
Outline how one calcium compound in the lime cycle can reduce a problem caused by acid deposition.
Markscheme
«nCaCO3 = =» 5.55 «mol» ✓
«V = 5.55 mol × 22.7 dm3 mol−1 =» 126 «dm3» ✓
Award [2] for correct final answer.
Accept method using pV = nRT to obtain the volume with p as either 100 kPa (126 dm3) or 101.3 kPa (125 dm3).
Do not penalize use of 22.4 dm3 mol–1 to obtain the volume (124 dm3).
«ΔH =» (−635 «kJ» – 393.5 «kJ») – (−1207 «kJ») ✓
«ΔH = + » 179 «kJ» ✓
Award [2] for correct final answer.
Award [1 max] for −179 kJ.
Ignore an extra step to determine total enthalpy change in kJ: 179 kJ mol−1 x 5.55 mol = 993 kJ.
Award [2] for an answer in the range 990 - 993« kJ».
lower activation energy curve between same reactant and product levels ✓
Accept curve with or without an intermediate.
Accept a horizontal straight line below current line with the activation energy with catalyst/Ecat clearly labelled.
provides an alternative «reaction» pathway/mechanism ✓
Do not accept “lower activation energy” only.
Ca(OH)2 (aq) + 2HCl (aq) → 2H2O (l) + CaCl2 (aq) ✓
«nHCl = 0.0350 dm3 × 0.025 mol dm−3 =» 0.00088 «mol»
OR
nCa(OH)2 = nHCl/0.00044 «mol» ✓
«V = =» 0.029 «dm3» ✓
Award [2] for correct final answer.
Award [1 max] for 0.058 «dm3».
Alternative 1:
[OH−] = « 2 × 2.33 × 10−2 mol dm−3 =» 0.0466 «mol dm−3» ✓
«[H+] = = 2.15 × 10−13 mol dm−3»
pH = « −log(2.15 × 10−13) =» 12.668 ✓
Alternative 2:
[OH−] =« 2 × 2.33 × 10−2 mol dm−3 =» 0.0466 «mol dm−3» ✓
«pOH = −log (0.0466) = 1.332»
pH = «14.000 – pOH = 14.000 – 1.332 =» 12.668 ✓
Award [2] for correct final answer.
Award [1 max] for pH =12.367.
«nCa(OH)2 = 2.41 dm3 × 2.33 × 10−2 mol dm−3 =» 0.0562 «mol» AND
«nCO2 ==» 0.0330 «mol» ✓
«CO2 is the limiting reactant»
«mCaCO3 = 0.0330 mol × 100.09 g mol−1 =» 3.30 «g» ✓
Only award ECF for M2 if limiting reagent is used.
Accept answers in the range 3.30 - 3.35 «g».
« × 100 =» 86.4 «%» ✓
Accept answers in the range 86.1-86.4 «%».
Accept “71.3 %” for using the incorrect given value of 4.00 g.
«add» Ca(OH)2/CaCO3/CaO AND to «acidic» water/river/lake/soil
OR
«use» Ca(OH)2/CaCO3/CaO in scrubbers «to prevent release of acidic pollution» ✓
Accept any correct name for any of the calcium compounds listed.
Examiners report
This question is about peroxides.
Hydrogen peroxide decomposes to water and oxygen when a catalyst such as potassium iodide, KI, is added.
2H2O2 (aq) O2 (g) + 2H2O (l)
Suggest why many chemicals, including hydrogen peroxide, are kept in brown bottles instead of clear colourless bottles.
In a laboratory experiment solutions of potassium iodide and hydrogen peroxide were mixed and the volume of oxygen generated was recorded. The volume was adjusted to 0 at t = 0.
The data for the first trial is given below.
Plot a graph on the axes below and from it determine the average rate of formation of oxygen gas in cm3 O2 (g) s−1.
Average rate of reaction:
Additional experiments were carried out at an elevated temperature. On the axes below, sketch Maxwell–Boltzmann energy distribution curves at two temperatures T1 and T2, where T2 > T1.
Apart from a greater frequency of collisions, explain, by annotating your graphs in (b)(ii), why an increased temperature causes the rate of reaction to increase.
MnO2 is another possible catalyst for the reaction. State the IUPAC name for MnO2.
Comment on why peracetic acid, CH3COOOH, is always sold in solution with ethanoic acid and hydrogen peroxide.
H2O2 (aq) + CH3COOH (aq) CH3COOOH (aq) + H2O (l)
Sodium percarbonate, 2Na2CO3•3H2O2, is an adduct of sodium carbonate and hydrogen peroxide and is used as a cleaning agent.
Mr (2Na2CO3•3H2O2) = 314.04
Calculate the percentage by mass of hydrogen peroxide in sodium percarbonate, giving your answer to two decimal places.
Markscheme
decomposes in light [✔]
Note: Accept “sensitive to light”.
points correctly plotted [✔]
best fit line AND extended through (to) the origin [✔]
Average rate of reaction:
«slope (gradient) of line =» 0.022 «cm3 O2 (g) s−1» [✔]
Note: Accept range 0.020–0.024cm3 O2 (g) s−1.
peak of T2 to right of AND lower than T1 [✔]
lines begin at origin AND T2 must finish above T1 [✔]
Ea marked on graph [✔]
explanation in terms of more “particles” with E ≥ Ea
OR
greater area under curve to the right of Ea in T2 [✔]
manganese(IV) oxide
OR
manganese dioxide [✔]
Note: Accept “manganese(IV) dioxide”.
move «position of» equilibrium to right/products [✔]
Note: Accept “reactants are always present as the reaction is in equilibrium”.
M (H2O2) «= 2 × 1.01 + 2 × 16.00» = 34.02 «g» [✔]
«% H2O2 = 3 × × 100 =» 32.50 «%» [✔]
Note: Award [2] for correct final answer.
Examiners report
The explanation that the brown bottle prevented light causing a decomposition of the chemical was well answered but some incorrectly suggested it helped to stop mixing up of chemicals e.g. acid/water/peroxide.
The graphing was disappointing with a surprising number of students missing at least one mark for failing to draw a straight line or for failing to draw the line passing through the origin. Also some were unable to calculate the gradient.
The drawing of the two curves at T1 and T2 was generally poorly done.
Explaining why temperature increase caused an increase in reaction rate was generally incorrectly answered with most students failing to mention “activation energy” in their answer or failing to annotate the graph.
Many could correctly name manganese(IV)oxide, but there were answers of magnesium(IV) oxide or manganese(II) oxide.
Suggesting why peractic acid was sold in solution was very poorly answered and only a few students mentioned equilibrium and, if they did, they thought it would move to the left to restore equilibrium.
Calculating the % by mass was generally well answered although some candidates started by using rounded values of atomic masses which made their final answer unprecise.
Sulfur trioxide is produced from sulfur dioxide.
2SO2 (g) + O2 (g) 2SO3 (g) ΔH = −196 kJ mol−1
The reaction between sulfur dioxide and oxygen can be carried out at different temperatures.
Nitric acid, HNO3, is another strong Brønsted–Lowry acid. Its conjugate base is the nitrate ion, NO3−
Outline, giving a reason, the effect of a catalyst on a reaction.
On the axes, sketch Maxwell–Boltzmann energy distribution curves for the reacting species at two temperatures T1 and T2, where T2 > T1.
Explain the effect of increasing temperature on the yield of SO3.
State the product formed from the reaction of SO3 with water.
State the meaning of a strong Brønsted–Lowry acid.
Draw the Lewis structure of NO3−.
Explain the electron domain geometry of NO3−.
Markscheme
increases rate AND lower Ea ✔
provides alternative pathway «with lower Ea»
OR
more/larger fraction of molecules have the «lower» Ea ✔
Accept description of how catalyst lowers Ea for M2 (e.g. “reactants adsorb on surface «of catalyst»”, “reactant bonds weaken «when adsorbed»”, “helps favorable orientation of molecules”).
both axes correctly labelled ✔
peak of T2 curve lower AND to the right of T1 curve ✔
lines begin at origin AND correct shape of curves AND T2 must finish above T1 ✔
Accept “probability «density» / number of particles / N / fraction” on y-axis.
Accept “kinetic E/KE/Ek” but not just “Energy/E” on x-axis.
decrease AND equilibrium shifts left / favours reverse reaction ✔
«forward reaction is» exothermic / ΔH is negative ✔
sulfuric acid/H2SO4 ✔
Accept “disulfuric acid/H2S2O7”.
fully ionizes/dissociates ✔
proton/H+ «donor » ✔
Do not accept the delocalised structure.
Accept any combination of dots, crosses and lines.
Coordinate/dative bond may be represented by an arrow.
three electron domains repel
OR
three electron domains as far away as possible ✔
trigonal planar
OR
«all» angles are 120° ✔
Examiners report
A generally well-answered question. Most candidates explained the effect of a catalyst on a reaction correctly. A small proportion of candidates thought the catalyst increased the frequency of collisions. Some candidates focussed on the effect of the catalyst on an equilibrium since the equation above the question was that of a reversible reaction. These candidates usually still managed to gain at least the first marking point by stating that both forward and reverse reaction rates were increased due to the lower activation energy. Most candidates mentioned the alternative pathway for the second mark, and some gave a good discussion about the increase in the number of molecules or collisions with E≥Ea. A few candidates lost one of the marks for not explicitly stating the effect of a catalyst (that it increases the rate of the reaction).
The average mark scored for the Maxwell-Boltzmann distribution curves sketch was 1.5 out of 3 marks and the question had a strong correlation with the candidates who did well overall. The majority of candidates were familiar with the shapes of the curves. The most commonly lost mark was missing or incorrect labels on the axes. Sometimes candidates added the labels but did not specify “kinetic” energy for the x-axis. As for the curves, some candidates reversed the labels T1 and T2, some made the two curves meet at high energy or even cross, and some did not have the correct relationship between the peaks of T1 and T2.
Another question that showed a strong correlation with the candidates who did well overall. The average mark was 1 out of 2 marks. Many candidates explained the effect of an increase in temperature on the yield of SO3 correctly and thoroughly. One of the common mistakes was to miss the fact that it was an equilibrium and reason that yield would not change due to an increase in the rate of reaction. Unfortunately, a number of candidates also deduced that yield would increase due to the increase in rate. Other candidates recognized that it was an exothermic reaction but deduced the equilibrium would shift to the right giving a higher yield of SO3.
A very well answered question. 70% of the candidates stated H2SO4 as the product from the reaction of SO3 with water.
While a straightforward question, many candidates only answered part of the question - either focussing on the “strong” or on the “Brønsted-Lowry acid”. The average mark on this question was 1.2 out of 2 marks.
Only 20% of the candidates scored the mark for the Lewis structure of NO3-. Mistakes included: missing charge, missing lone pairs, 3 single bonds, 2 double bonds.
The majority of candidates deduced the correct electron domain geometry scoring the first mark including cases of ECF. Only a small number of candidates satisfied the requirements of the markscheme for the explanation.
Copper forms two chlorides, copper(I) chloride and copper(II) chloride.
An electrolysis cell was assembled using graphite electrodes and connected as shown.
State the electron configuration of the Cu+ ion.
Copper(II) chloride is used as a catalyst in the production of chlorine from hydrogen chloride.
4HCl (g) + O2 (g) → 2Cl2 (g) + 2H2O (g)
Calculate the standard enthalpy change, ΔHθ, in kJ, for this reaction, using section 12 of the data booklet.
The diagram shows the Maxwell–Boltzmann distribution and potential energy profile for the reaction without a catalyst.
Annotate both charts to show the activation energy for the catalysed reaction, using the label Ea (cat).
Explain how the catalyst increases the rate of the reaction.
Solid copper(II) chloride absorbs moisture from the atmosphere to form a hydrate of formula CuCl2•H2O.
A student heated a sample of hydrated copper(II) chloride, in order to determine the value of . The following results were obtained:
Mass of crucible = 16.221 g
Initial mass of crucible and hydrated copper(II) chloride = 18.360 g
Final mass of crucible and anhydrous copper(II) chloride = 17.917 g
Determine the value of .
State how current is conducted through the wires and through the electrolyte.
Wires:
Electrolyte:
Write the half-equation for the formation of gas bubbles at electrode 1.
Markscheme
[Ar] 3d10
OR
1s2 2s2 2p6 3s2 3p6 3d10 ✔
ΔHθ = ΣΔHθf (products) − ΣΔHθf (reactants) ✔
ΔHθ = 2(−241.8 «kJ mol−1») − 4(−92.3 «kJ mol−1») = −114.4 «kJ» ✔
NOTE: Award [2] for correct final answer.
Ea (cat) to the left of Ea ✔
peak lower AND Ea (cat) smaller ✔
«catalyst provides an» alternative pathway ✔
«with» lower Ea
OR
higher proportion of/more particles with «kinetic» E ≥ Ea(cat) «than Ea» ✔
mass of H2O = «18.360 g – 17.917 g =» 0.443 «g» AND mass of CuCl2 = «17.917 g – 16.221 g =» 1.696 «g» ✔
moles of H2O = «=» 0.0246 «mol»
OR
moles of CuCl2 =«= » 0.0126 «mol» ✔
«water : copper(II) chloride = 1.95 : 1»
« =» 2 ✔
NOTE: Accept « =» 1.95.
NOTE: Award [3] for correct final answer.
Wires:
«delocalized» electrons «flow» ✔
Electrolyte:
«mobile» ions «flow» ✔
2Cl− → Cl2 (g) + 2e−
OR
Cl− → Cl2 (g) + e− ✔
NOTE: Accept e for e−.